Objective: To investigate the safety and efficacy of high-dose infusional gemcitabine combined with busulfan and cyclophosphamide (GBC) or melphalan (GBM) followed by autologous stem-cell transplantation (ASCT) in lymphoid malignancies.
Methods: We retrospectively analyzed 73 and 21 patients of lymphoma, who received GBC and GBM conditioning regimen with ASCT respectively in our center from May 2017 to April 2020. Gemcitabine (600mg·m-2·h-1×3h) was given on day -7 and -3, busulfan (105mg/ m2) from day -7 to -5, followed by cyclophosphamide (50mg/kg) or melphelan (60mg/m2) from day -3 to -2. Autologous stem cells were reinfused on day 0. The side effects, hematopoiesis recovery time, 3-year progression free survival (PFS) and 3-year overall survival (OS) were observed.
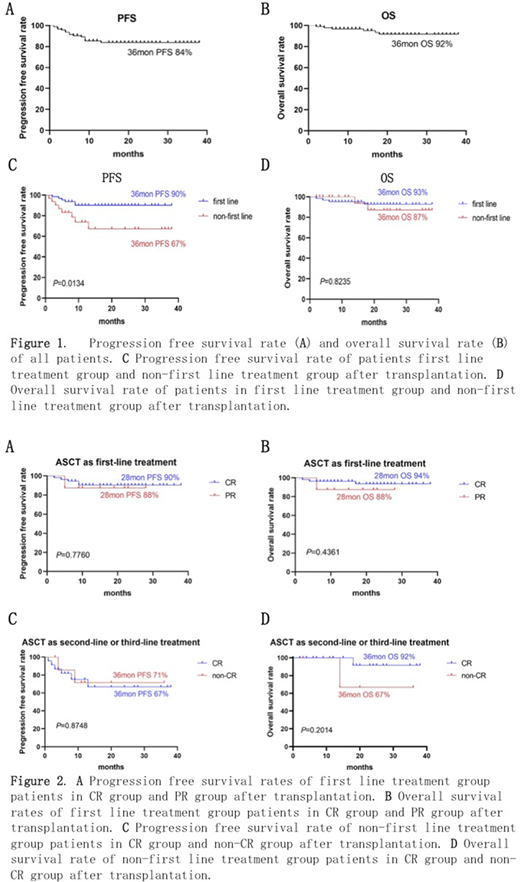
Results: Ninety-four patients were enrolled in this study. Among them, 63 cases (67%) were in the first line treatment, including 55 cases in CR1 and 8 cases in PR1, while 31 cases (33%) were in or after second line treatment, including 23 cases in CR, 7 cases in PR and 1 case in SD. There were 32 cases of large B-cell lymphoma (LBCL), 14 cass of mantle cell lymphoma (MCL) and 8 cases of peripheral T cell lymphoma (PTCL) in the first line treatment group, while 12 cases of Hodgkin lymphoma (HL) and 10 cases of LBCL in the non-first line treatment group. A median number of 3.09 (range 0.71-21.33) ×106/kg CD34 cells were infused. The grade 3 or 4 toxicities were neutropenia (100%), thrombocytopenia (100%), anemia (81%), oral mucositis (45%), hepatopathy (23%), nausea (14%), diarrhea (20%), vomiting (5%), hemorrhage (4%) and fever (1%). Bacterial infection was found in 9 cases (10%), and fungal infection in 2 cases (4%). The median time to neutrophil engraftment was 10 days (range, 8-28) and platelet engraftment was 11 days (range, 0-63). VOD and treatment-related death did not occur during the study. After a median follow-up of 21 months, the estimated 3-year PFS and OS rate of all patients was 84% and 92% respectively. The estimated 3-year PFS rates of patients in the first-line treatment group and the non-first line treatment group were 90% and 67%, respectively (P=.0134), while the estimated 3-year OS rates were 93% and 87%, respectively (P=.8235). The estimated 28-month PFS rates of first line treatment patients in the CR group and PR group were 90% and 88%, respectively (P=.7760), while the estimated 28-month OS rates were 94% and 88%, respectively (P=.4361). The estimated 3-year PFS rates of non-first line treatment patients in the CR group and PR group were 71% and 67%, respectively (P=.8748), while the estimated 3-year OS rates were 92% and 67%, respectively (P=.2014).
Conclusion: In this retrospective analysis, our results demonstrate that GBC/GBM conditioning regimens with ASCT are feasible with tolerable toxicity and improved outcomes in PR patients whether in first line or non-first line treatment, which appears be an alternative to the classical conditioning regimens for ASCT in lymphoid malignancies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal